Biomolecules labelled with positron-emitting radionuclides, such as fluorine-18, copper-64 or zirconium-89, are increasingly being used in nuclear medicine for diagnostic purposes, particularly in positron emission tomography (PET).
Radiolabelling is not a trivial process with two major requirements:
- the fragility and complexity of the biomolecules to be labelled mean that it is necessary to work under gentle conditions;
- their tertiary structure must be affected as little as possible by the process, at the risk of compromising their biological activity.
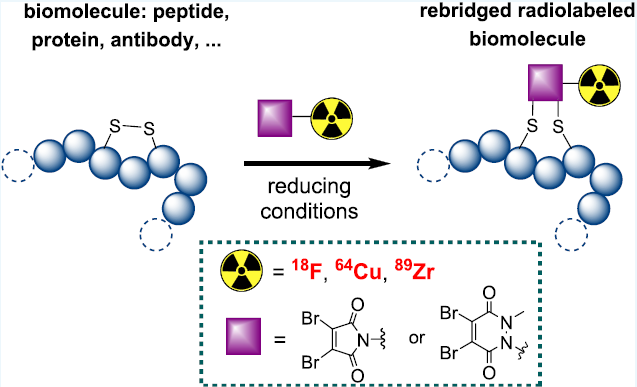
Disulfide bridges are a promising possibility as they would allow both requirements to be met.
In this context, researchers at the SHFJ have developed an original molecule for fluorine-18 labelling by 're-positioning' biomolecules containing disulphide bridges: the radiofluorinated prosthetic group dibromopyridazine dione. They used their molecule to radiolabel with [18F]:
- octreotide, a somatostatin analogue containing a disulphide bridge
- a fragment of an anti-PD-L1 antibody, a preferred target in the treatment of cancer by immunotherapy. This fragment is the result of work carried out at SIMoS.
© M. Richard et al. / Bioconjugate Chem. 2023, 34, 2123−2132
They also evaluated the properties of radiolabelled biomolecules in vitro and in vivo using positron emission tomography (PET) imaging.
The researchers then developed other rebinding molecules carrying the appropriate chelators, so that they could apply their strategy to radiolabelling with copper-64 or zirconium-89. This enabled them to label cetuximab, a monoclonal antibody. The use of isotopes could be extended to b emitters such as lutecium-177 for internal vectorised radiotherapy, opening up new theranostic applications.
Contact within the Frédéric-Joliot Institute for Life sciences: