Many therapeutic monoclonal antibodies are now currently used in cancer immunotherapy. In the coming years, a considerable number of targeted cancer therapies based on the use of such antibodies should become available. In order to select the best therapy, it is crucial to perform a molecular analysis of cancer cells and quantify as many protein biomarkers as possible, signatures of the tumor lesion. The iterative use of fluorescent antibodies to quantify these biomarkers in vitro is quite widely used (cyclic immunofluorescence), but remains limited by the number of fluorophores that can be detected.
In this manuscript, the authors describe the development of a novel strategy for multiplexed analysis of tumor cells and tissues in vitro and in vivo by applying their unique bioorthogonal cleavage chemistry on antibodies labeled with TMPP, a mass spectrometry tag (MS-tag), from which it is theoretically possible to generate 61 isotopologues. The researchers selected four TMPP isotopologues and linked them to four FDA-approved therapeutic antibodies via laboratory-developed sydnonimine cleavable links (SPICC). Following a bioorthogonal “click-and-release" reaction, in which the MS-tag is released in favor of the attachment of a fluorescent compound, it was possible to localize all tumor antigen receptors (fluorescent labeling), and to identify and quantify the 4 receptors by mass spectrometric analysis of the cleaved and released MS-tags. Compared with cyclic immunofluorescence, this strategy does not provide spatial information on individual receptors, but does offer i) a greater number of cancer biomarkers detected, ii) the (semi-) quantification of the expression levels of these biomarkers, and iii) the possibility of using this technology in vivo.
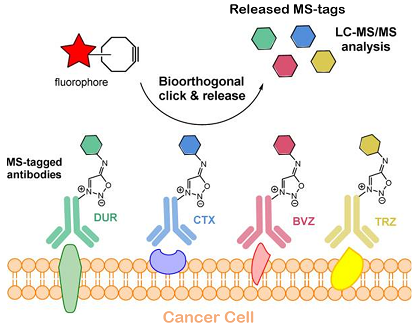
Immunological profiling of cancer: the combination of bioorthogonal chemistry and LC-MS/MS analysis successfully used for imaging and quantification of four cancer receptors overexpressed in cells, tissues and in vivo.
Ribéraud et al, Chem Sci 2024
The originality of the approach described in this study lies in the novel combination of two technologies: the design of chemically cleavable links on a cocktail of anti-cancer antibodies, and the multiplexed mass spectrometric detection of several MS-tags specific to each antibody. This promising approach has enabled the detection of four tumor markers in cultured cells, biopsies and in a tumor-bearing animal model.
-
Tumor immunoprofiling: detection of the presence of certain antigens overexpressed by cancer cells using antibodies
-
TMPP (tris(2,4,6,-trimethoxyphenyl)-phosphonium) is a highly suitable MS-tag for proteomic studies. It has 9 methoxy groups, 6 hydrogens and 27 carbon atoms, making it ideal for deuterium or carbon-13 tagging. A total of 60 atoms can be isotopically labelled, theoretically generating 61 isotopologues.
-
SPICC : In 2017, SCBM researchers discovered that iminosydnones (mesoionic reagents) are ideal reagents for click-and-release reactions. Grafted onto a protein, they react rapidly with cyclic alkynes present in biological media to form a first product resulting from the ligation of the 2 reaction partners (protein + cyclic alkyne), then a second corresponding to the release of the iminosydnone from the protein (
strain-promoted iminosydnones-cycloalkyne cycloaddition, SPICC). See
Joliot news