It's well known that plants photosynthesize using solar energy, and that this leads to the formation of oxygen. What is less known is that this complex process is highly regulated, and that plants are constantly optimizing their molecular strategies to adapt to the light conditions of their environment. The photosynthetic apparatus of plants is made up of two protein supercomplexes that function in series, photosystems I and II (PSI and PSII). Each complex, by capturing light, triggers a photochemical reaction: photosystem II, the first of the two centers involved in photosynthesis, is a highly oxidizing system, leading to the formation of oxygen (O2); photosystem I, on the other hand, produces electrons with high reducing power. One of the compounds reduced by PSI is O2, which is transformed into superoxide anion (O2•−), a Reactive Oxygen Species (ROS) that can be both deleterious to the photosynthetic apparatus and beneficial as a cell-signaling molecule. Thus, O2 reduction at PSI acts as a regulator, sensitive to changes in the light environment. At the heart of this process, the activity of PSI redox enzymes is crucial in controlling the quantities of produced ROS and maintaining adequate photosynthetic function. However, the respective role of each enzyme and its interactions with partners within the PSI remain poorly understood.
In this work, the authors finely studied the regulation of O2 reduction to O2•− in the PSI in the model plant Arabidopsis thaliana grown during different photoperiods (alternating short and long days) and in different mutants, using electron paramagnetic resonance (EPR) measurements to detect O2•−. Knowing that the main redox enzymes involved in the control of ROS generated in chloroplasts during photosynthesis are m-type thioredoxins, NADPH-dependent reductase C (NTRC) and 2-Cys peroxiredoxin (2-Cys PRX), the authors investigated whether these SH-dependent enzymes interact with each other and can function as a redox regulatory network. In wild plants grown on short days, ROS generation is 2 times higher than in samples grown on long days, and this difference is suppressed in several redoxin mutants. Thioredoxin m and 2-Cys-PRX localization on the thylakoid membrane varies with photoperiod. In vitro experiments indicate that several enzymes are required to achieve a strong O2 reduction in thylakoids of plants grown on long days. Thus, the interaction of these enzymes effectively functions as a redox regulatory network in which 2-Cys PRX can be reduced by NTRC and Thioredoxin, but can also re-oxidize reduced thioredoxins, thus providing a rapid adaptation system to changes in light regime.
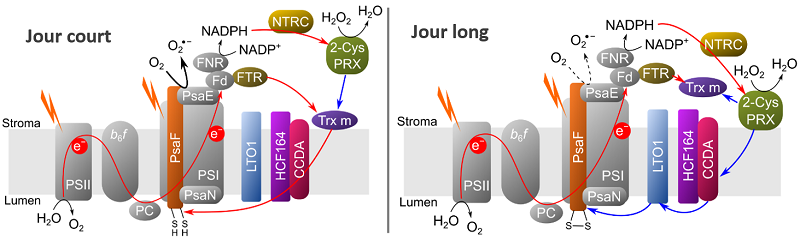
Redox regulation model of O2 reduction in photosystem I.
Red arrows indicate photosynthetic electron flow. Black arrow indicates strong short-day O2 reduction, dashed black arrow indicates weak long-day O2 reduction. Hani et al, Plant Physiol, 2024
Taken together, these results have led the researchers to propose a new model for the redox regulation of O2 reduction at PSI, depending on the reducing power of the chloroplast interior (stroma) and the ability of the various thiol-containing proteins to form a network of redox interactions. This model could guide new research into the redox regulation of alternative electron transport pathways under conditions of fluctuating light or abiotic stress (drought, excess water, extreme temperatures, pollution...).
Contact : Anja Liszkay (anja.liszkay@i2bc.paris-saclay.fr ; anja.krieger-liszkay@cea.fr)
A thylakoid is a membrane compartment within chloroplasts where light-dependent reactions take place during photosynthesis. Thylakoids consist of a membrane surrounding a lumen.