There is a lot happening inside a lithium-ion battery cell at any given time. For battery specialists, the ability to observe—in real time—parameters like temperature, pressure, electrochemical potential, lithiation kinetics, structural heterogeneities, and outgassing would be a game changer.
Access to real-time data could provide new insights into variations in battery performance and the reasons behind deterioration; the early detection of thermal runaway could improve safety; and lifespans could be extended if knowledge of the optimal cycles and current regimes were available. And then there is recycling: with information about a battery's service life, second-life and recycling options could be evaluated more effectively.
Sensors inside battery cells subjected to extreme conditions
There are only two ways to find out what is happening inside a battery in real time. The first is to place a sensor inside the cell. The second is to map the battery using X-ray imaging. Sensor experts at CEA-Liten and X-ray experts at CEA-Irig combined the two in a unique synchrotron experiment for this research. The results of the experiment, which was a collaborative effort by the European BIGMAP and INSTABAT project consortia, were published in
Nature Communications.
Instrumenting battery cells with sensors is more complicated than it looks. The very presence of the sensor can alter the process it is intended to measure. Not to mention the conditions inside a lithium-ion battery. From electric fields to continuous electrochemical reactions, this environment is hard on sensors. Finally, the ability to look inside the “black box" is crucial to manufacturers, who need guarantees that sensor measurements accurately reflect what is really happening inside the cell.
X-ray imaging of an optical fiber and a reference electrode
CEA-Liten sensor experts instrumented a commercial multi-layer 1.1 Ah lithium-ion battery cell with two sensors: a thermoluminescence-based optical fiber (to measure internal temperature), and a reference electrode (to measure variations in anode and cathode potentials).
The instrumented cell was examined on a beamline at ESRF, the European Synchrotron Radiation Facility, Grenoble, France. X-ray diffraction was used to observe the graphite lithiation and delithiation processes at work inside the cell. Synchrotron
operando experiment expert Sandrine Lyonnard of CEA-Irig, who co-authored the paper in
Nature Communications, pointed out the growing popularity of the technique. “Batteries are heterogeneous systems in which determining the changes in materials at the atomic scale and mapping structures throughout the entire thickness of a component are both important." The multidisciplinary team took
operando measurements during different cycling and current regimes over a period of four hours. Another of the paper's co-authors, CEA-Liten physicist and sensor expert Olivier Raccurt, said, “This is the most detailed observation of an instrumented lithium-ion battery cell during operation to date." It took a year to process and analyze the real-time measurement data. But the results were worth it.
Optical fiber deforms stack
CEA-Irig PhD candidate Annabel Olgo, the paper's first author, analyzed the data. “We were able to show that lithiation was slower in the immediate vicinity of the optical fiber. The impact of the optical fiber was very local and did not negatively impact electrochemical performance over the duration of our experiment. It could be that this alteration occurs after a longer lapse of time. It could also be that the build-up and metallization of the lithium cause a short circuit. Aging tests would be needed to confirm this."
Geometry could explain the phenomenon: The 225-micrometer-diameter optical fiber, when inserted between two layers of the electrode material, deforms the stack. The reference electrode, which, at just a few micrometers thick, has little to no effect on local lithiation.
A battery instrumentation design and impact testing tool
The results of this research could help battery manufacturers—many of whom are developing their own sensors now—determine the best sensor geometries, positions within the cell, and impacts on electrochemical reactions, the electrolyte, and electrodes. Graphite is known to degrade faster than the cathode material, for example.
Raccurt explained, “We have developed an observational tool that can be used to evaluate a specific sensor prototype and to answer broader questions about the best approach and the optimal choices for instrumenting a battery cell. There is no such thing as the perfect
in-situ sensor, but our new tool will help battery stakeholders get closer to effective solutions."
X-ray diffraction a powerful observational tool
This research also confirmed the major role synchrotron beamlines can play in developing smart battery monitoring systems. CEA-Irig's researchers pushed the limits of what they thought was possible to effectively deploy X-ray diffraction for this experiment. Lyonnard said, “The centimeter-thick battery cell consisted of several rolls of the different constituent parts. We had to have an X-ray beam with high enough energy to pass through this system, but small enough—50 microns—to probe specific points. The beam also had to be compatible with the high-speed acquisition of high-quality diffraction data."
Looking ahead, sophisticated correlative measurement techniques like this one could be combined with tomography to enable the kinds of 3D measurements that could help scientists and engineers better monitor, control, and repair batteries.
* Institute of Chemistry, University of Tartu (Estonia), ESRF, Institut Néel, LEPMI-CNRS
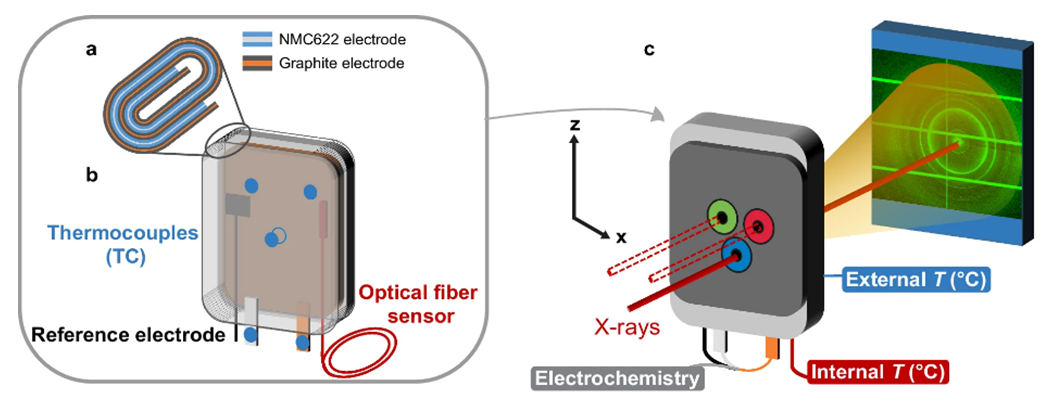
Fig 1: Schematic diagram of the experiment. Cell parameters: external (thermocouples) and internal (optical fiber) temperatures, cell potential, potential of each electrode (internal reference electrode) (left). DRX measurement showing the impact of the optical fiber on graphite lithiation during high-speed charging (2C) (right).
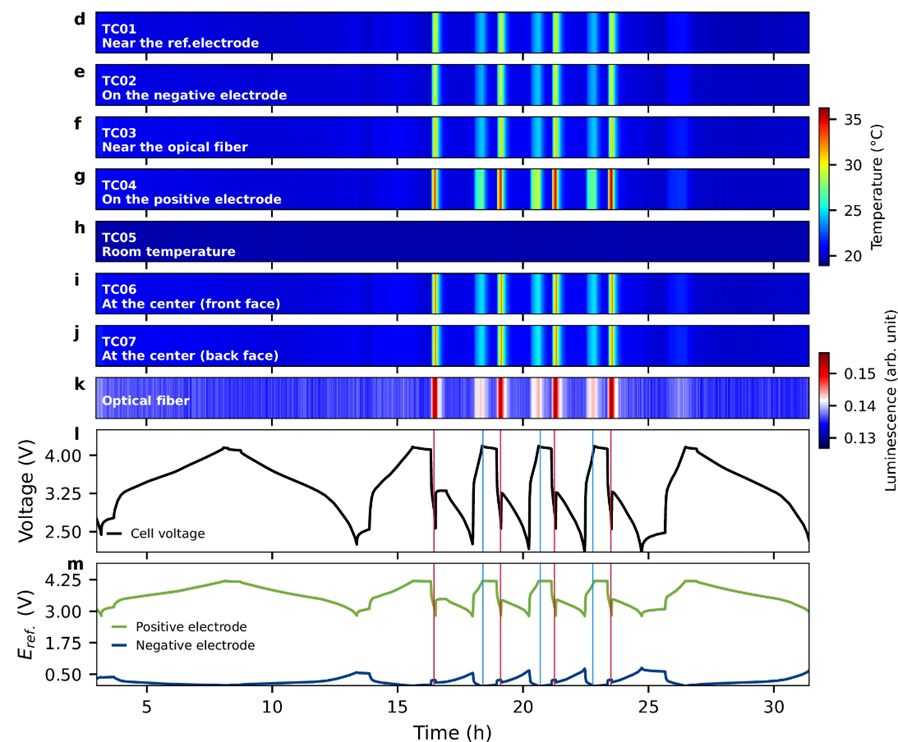
Fig 2: Results of operando and in-situ measurements on a 1.1 Ah Lithium ion pouch cell.