Proteins containing iron-sulfur (Fe-S) clusters are involved in many cellular processes, such as DNA replication and repair, respiration and photosynthesis. Their importance lies in their functional versatility, including electron transfer, redox and non-redox catalysis, regulation of gene expression and the supply of sulfur atoms. These Fe-S clusters do not form spontaneously in vivo, but require complex multi-protein machineries, known as Fe-S factories, to assemble them.
Researchers at CEA-IRIG have long focused on understanding these factories that preform Fe-S clusters before transferring them to target proteins in the cell, such as respiratory complexes. The exact nature and ligands of the Fe-S clusters within these factories remain a mystery, despite recent advances in biophysics and genetics.
In this article, the researchers [Collaboration] studied the SufBC2D Fe-S cluster factory in bacteria. Using a native purifiedsystem , they characterized this factory containing natural Fe-S clusters. Using biophysical characterization techniques such as X-ray absorption spectroscopy, Mössbauer spectroscopy, Electron paramagnetic resonance spectroscopy and UV-visible absorption spectroscopy, they found that SufBC2D mainly contains a [2Fe-2S]-type cluster, and an unidentified species, possibly a [3Fe-5S]-type cluster.
By analyzing protein variants, the researchers identified several amino acids involved in the coordination of the [2Fe-2S] cluster, suggesting a coordination at the interface of the SufB and SufD proteins. This study provides new information on the molecular organization of Fe-S clusters in the SufBC2D factory and raises questions about the nature of the second iron-sulfur species observed, a topic to be further explored in the context of Fe-S cluster biosynthesis and more broadly in bioinorganic chemistry.
This work will provide a better understanding of the mechanisms by which Fe-S clusters are formed
in vivo with a view to opening up new avenues for biotechnological and medical applications.
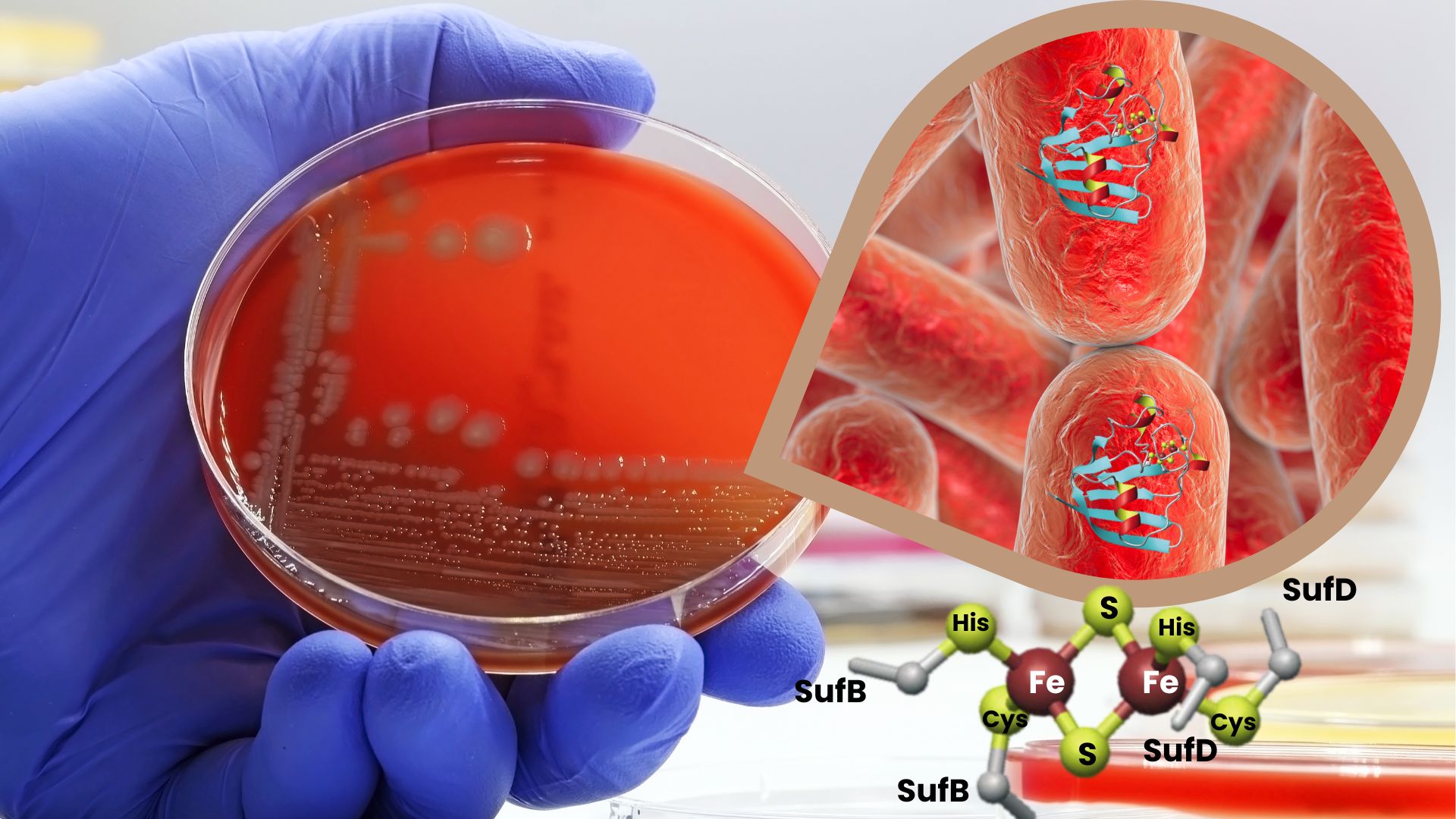
Figure: Study of the SufBC2D factory in bacteria
E. coli (© CEA)
Collaboration
- LCBM / BioCat, PMB, ComX
- IRIG/SYMMES, CAMPE
- Institut Pasteur/Unit Stress Adaptation and Metabolism in enterobacteria
- LCB, Marseille
Fundings
- French ANR grant FeStreS (2012-2016)
- French ANR grant MASTIC (2023-2027)