Morphogenesis encompasses all the mechanisms that induce the appearance of forms from initially homogeneous systems. Revealed by chemistry and physics, these mechanisms are responsible for the appearance of regular structures in biology. A new morphogenesis has just been discovered by studying the self-organization of microtubules.
By reconstituting an in vitro system with molecular motors directed at both ends of the microtubules, the researchers discovered that these motors not only moved the microtubules, but also aligned them, forming “barriers”. At the same time, these motors separated into distinct domains, creating an ordered yet dynamic structure where microtubules and motors influence each other. This organization is constantly evolving, changing shape or disappearing according to the forces implicated.A theoretical approach equated the necessary balance between transport and diffusion of molecular motors, determining the precise conditions for the appearance of regular patterns, and revealed that an imbalance in motor concentration, and therefore in the forces exerted on microtubules, prevented pattern formation, causing constant microtubule movement. It also identified the exact conditions under which a slight variation in motor concentration caused the experimental system to switch from constant microtubule movement to sudden immobilization, resulting in the appearance of alternating domains.
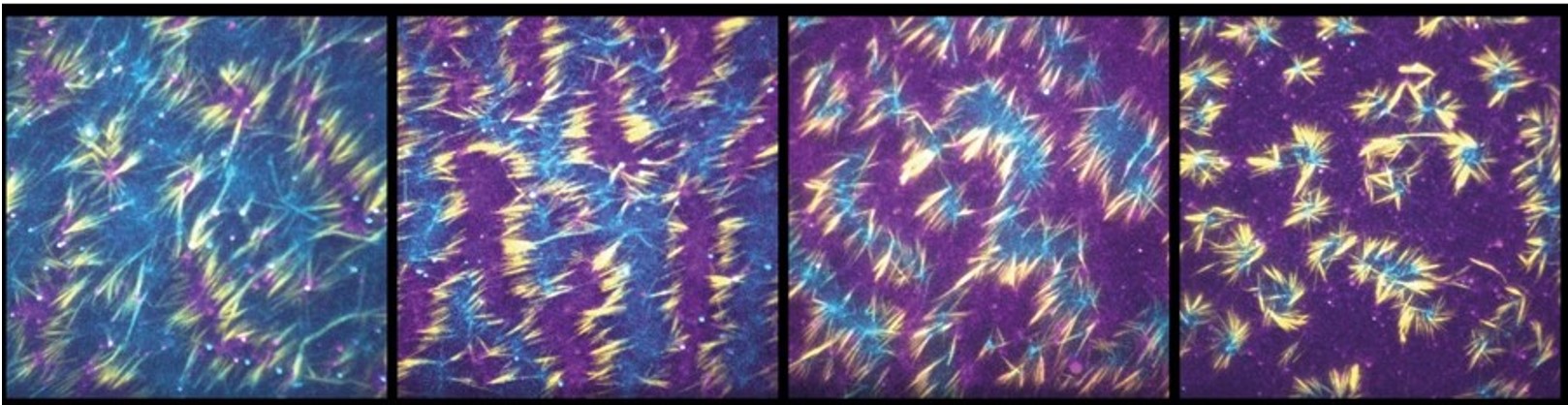
Superposition of images obtained by TIRF microscopy (evanescent wave imaging) to visualize microtubules (yellow), and two molecular motors: KIF5B (magenta) and NCD (cyan) at different concentrations. From left to right, KIF5B concentrations increase from 1 to 4 nM, while NCD concentrations decrease from 6 to 1 nM. Microtubule density is around 1 microtubule per µm2.
Mirroring the self-organization observed in vitro, microtubules often align in cells with polarities oriented in the same direction, and motors of the same polarity tend to form more concentrated domains in line with their direction of travel.
This hypothesis could challenge current models of the mechanisms by which cells define their orientation axes. This work opens up new perspectives on the internal organization of cells.
Collaborations
CEA/IRIG
CEA/ESPCI
CNRS/LPCVCNRS/PCC
Collège de France
Fundings
ANR Sharp AAPG2022-PRC-SHARP awarded to Manuel Théry and Jean-François Joanny.
ANR Sensation ANR-23-CHBS-0013 France 2030 awarded to Manuel Théry.