Diatoms are very abundant oceanic organisms that contribute up to 20% of the daily planetary carbon assimilation. This function is mediated by their pyrenoid, a structure that concentrate the CO2 fixing enzyme Rubisco inside the PyShell, a protein barrier that modulates gas exchanges with the cell. By concentrating Rubisco inside a specific compartment, the pyrenoid increases the efficiency of CO2 assimilation in an oxygen-free environment.
Despite the importance of this process, the molecular mechanism allowing diatoms to efficiently assimilate CO2 via their pyrenoids remains poorly understood. In this study a French, Japanese and Swiss consortium combine state of the art imaging and photophysiology to characterise the pyrenoid shell (PyShell) layer in vivo. This proteinaceous ordered assembly is localized at the pyrenoid periphery of Diatoms.
Using in situ cryo-electron tomography (cryo-ET), single particle cryo-ET and Focused Ion Beam Scanning Electron Microscopy (FIB-SEM), researchers reveal that the PyShell encased in a lattice-like protein sheath -instead of a lipid membrane. Disruption of the PyShell protein sheath by targeted mutagenesis leads to a fragmented pyrenoid structure, high-CO2 requiring-photosynthesis and reduced cell growth. Recombinant PyShell proteins self-assembled into helical tubes enabling the researchers to determine a 3.0 Å-resolution PyShell structure and fit it into the in vivo structure.
Overall the structure and function of the diatom PyShell provides new molecular insights for how CO2 is assimilated in the ocean, a crucial biome to mitigate climate change. Diatoms are marine organisms that assimilate almost 20% of the world's carbon on a daily basis. Thus, the study of their pyrenoid open new insights into the mechanism of this greenhouse gas assimilation in the ocean.
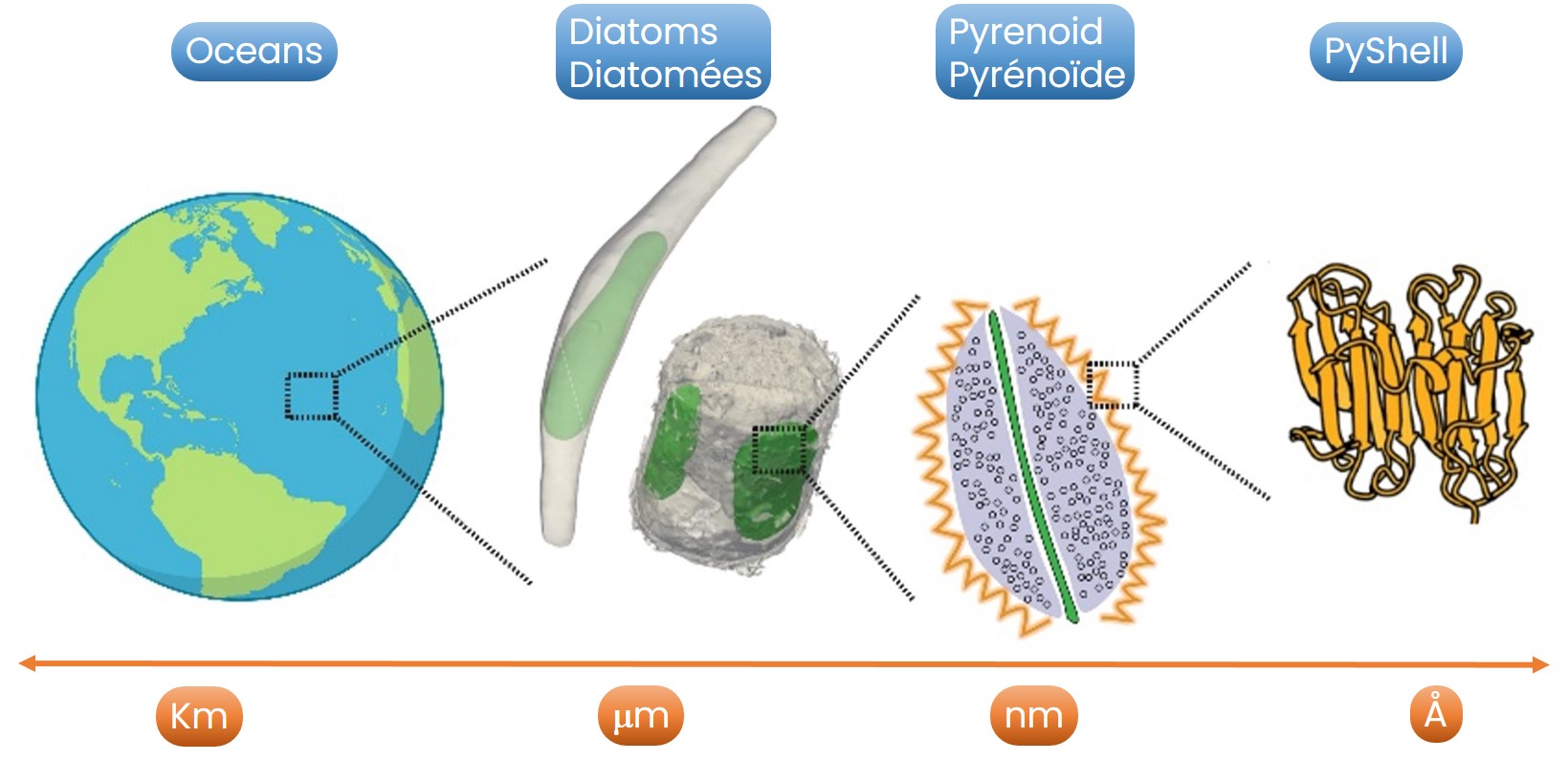
Figure: Focus on the diatoms PyShell (credit CEA).