Organoids are proving remarkably useful for evaluating the therapeutic efficiency of drugs or new molecules. However, they need to be vascularised to promote the exchange and transport of nutrients and oxygen, otherwise their maturation and growth are impaired.
In vivo the vascularisation is ensured by blood flow.
Researchers at IRIG and LETI have succeeded in vascularising organoids
in vitro on a microfluidic chip at speeds and flow rates similar to those of blood. The researchers' innovative idea was first to develop a self-organising vascular network within the chip, and then to trap an organoid containing its own endothelial cells within it, which would connect to the existing network and enable the organoid to be perfused
in vitro, mimicking the blood system.
Moreover, the innovative architecture of the microfluidic chip enables the organoids to be anchored: instead of randomly depositing the organoid on the chip, it is fixed in a single alveolus enabling precise observation of its development and exchanges with the endothelial network. The anchoring success rate is 95%. In addition, to compensate for the great variability of 3D cellular aggregates, this anchoring is selective by adjusting the size of the alveoli (cf.
images B-E).
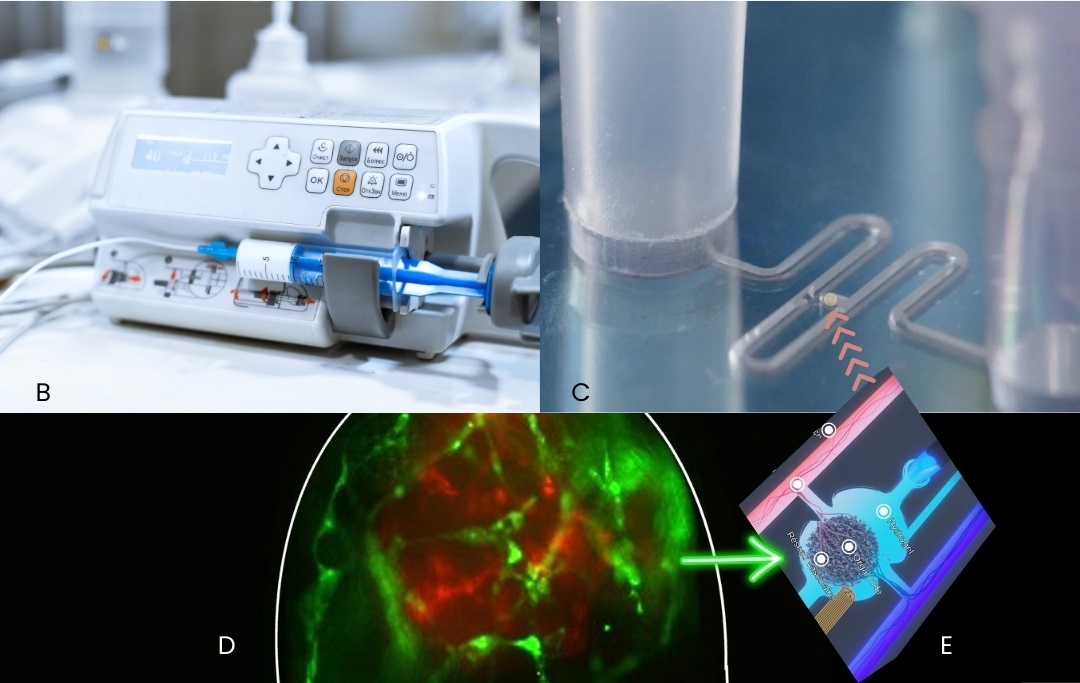
Images:(B) emulating vascularisation; (C) microfluidic channel; (D) fluorescence image of vascular network ; (E) localised implantation scheme in the microfluidic chip. Credit CEA
By vascularizing organoids
in vitro on a microfluidic chip, and maintaining them in culture for 30 days, researchers have for the first time in the world observed a significant improvement in their growth, maturation and physiological functions, virtually equivalent to those observed after xenotransplantation in mice. This technology represents a significant advance and offers an alternative to animal experimentation.
Organoid is an assembly of self-organising 3D cells capable of partially mimicking different physiological characteristics of an organ or tissue. The suffix "oid" means "resembling". An organoid is an
in vitro human model and is therefore not a mini-organ.