The bacterium
Staphylococcus aureus is one of the pathogens responsible for hospital-acquired pneumonia that has become resistant to antibiotics. Researchers are studying an alternative treatment using Lysostaphin enzyme. The enzyme bacteriolytic property enables to target the vital integrity of
S. aureus by reacting with the peptidoglycan polymer lining the bacterial wall.
To understand how it works precisely, researchers at IRIG used two enzymes of the same family Lysostaphin and LytM that are evolutionarily related but have different functions
The Irig team studied the high lytic activity of the enzymes Lysostaphin and LytM against the bacterium
S. aureus, by targeting the glycil-glycine bridge linking the peptide strands of the bacterial wall polymer. Monitoring of the catalytic reaction revealed these two enzymes acted in a similar way on the polymer fragments but only Lysostaphin was capable of solubilising the entire polymer .
Analysis of the nuclear magnetic resonance NMR characterization data led to coherent models for the docking of the enzymes on the polymer.
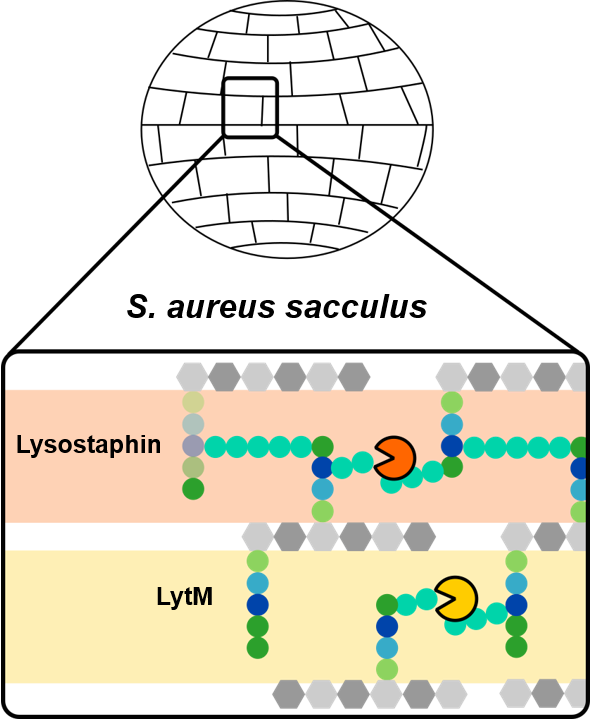
The researchers have shown that each enzyme reacts distinctly with the polymer depending on the complexity of
cross-linking and therefore has different biological functions (
cf. figure).
Figure: model of the activity of two enzymes LytM and Lysostaphin belonging to the same family but modulated differently by the state of cross-linking of bacterial peptidoglycan. Credit CEA
Using nuclear magnetic resonance NMR, combined with mass spectrometry, this work proposes a model in which peptidoglycan
cross-linking affects the activity and selectivity of the enzymes Lysostaphin and LytM differently, underpinning the specific role of each structurally related enzyme. These results highlight the complex interaction between enzymes and their substrates. They pave the way for targeted antibacterial strategies.
Cross-linking is a linear transformation into a three-dimensional polymer.